© Copyright 2002 by Paul Ramp. All rights reserved.
Chapter 35: The Respiratory System
Objectives:
- identify and describe the organs of the respiratory system
- describe the mechanism for oxygen transport
- describe the mechanism for carbon dioxide transport
- describe how your body regulates respiration
From this unit you should be able to:
Web Text:
- - Need for Gas Exchange -
- - Organs of the Respiratory System -
- - Mechanism of Breathing -
- - Gas Exchange in the Lungs -
- - Oxygen Transport -
- - Carbon Dioxide Transport -
- - Regulation of Respiration -
Need for Gas Exchange - In order for the cells of your body to conduct their metabolism, they require a source of energy. The energy source for the vast majority of the chemical reactions occurring in the cells is from the breakdown of a molecule called ATP. To replace the consumed ATP, your cells breakdown molecules such as glucose. For these reactions to produce enough ATP from glucose to sustain your life, your cells require a supply of oxygen. A waste product from these reactions producing ATP is carbon dioxide. It is the function of the respiratory system, in conjunction with the circulatory system, to deliver oxygen to each of the cells in your body and to carry away the carbon dioxide wastes before they reach toxic levels.
Organs of the Respiratory System - In essence, the respiratory system is composed of a series of tubes leading from the outside to small sacs within your chest. These small sacs are further enclosed in larger sacs, the lungs.
Air is drawn into the respiratory either through the nasal cavities or through the oral cavity. Most of the time we breathe through our nose that is specialized for this purpose. Within the nasal cavities are hairs and mucus membranes that trap dust particles, humidifies and warms the incoming air. The oral cavity is less efficient at conditioning the air we breathe in. This is why prolonged breathing through the mouth often leads to a dry, scratchy throat.
The nasal cavities and oral cavity join at the back of the mouth forming a common tube, the pharynx. The pharynx extends about halfway down your neck. At this point, the passageway splits with one tube (the esophagus) leading to the stomach while a second tube (the trachea) leads to the lungs. At this junction there is a flap of elastic cartilage called the epiglottis. While breathing, the epiglottis is in an upright position allowing air to enter the trachea. When food or liquids are swallowed, the epiglottis moves down, closing off the trachea and directing the swallowed items into the esophagus.
|
From the pharynx, air moves past the epiglottis and in the trachea. Connecting these two tubes is a short passageway called the larynx or voice box. Here, membrane folds are stretched across the passageway. As air moves past these folds, the folds vibrate. By controlling how tightly stretched the folds are, and by how open the passageway is, we are able to produce sounds. As these sounds resonate within the pharynx, nasal and oral cavities and modified by the muscles of the face, tongue and lips, we are able to make recognizable speech. The trachea is a tube about 4 to 5 inches in length and about one inch in diameter. Rings of cartilage support this passageway keeping it open for unobstructed airflow. The epithelium of the trachea is composed of pseudostratified columnar epithelial cells. The exposed surface of these cells are ciliated. Small dust particles that have penetrated this far can be trapped by this epithelium. The cilia move these particles up the trachea and into the pharynx where they are then swallowed. At the inferior end of the trachea, the tube splits into left and right branches. These are the bronchi. The bronchial tubes are also held open by cartilage rings. |
On entering the lungs, the bronchi branch again and again, the smaller tubes being called bronchioles. Ultimately, the bronchioles terminate in clusters of sacs called alveoli. The alveoli have very thin walls and are surrounded by capillaries. This is the site of gas exchange between the lungs and the blood. Its been estimated that the lungs contain 300 million alveoli. This provides a large surface area for gas exchange. It is estimated that the surface area of the lungs is roughly 750 square feet, about half the size of a modest home.
To aid the exchange of respiratory gasses between the air space within the alveoli and the surrounding capillaries, the alveoli produce a fluid coating called surfactant. This soap-like fluid lowers the surface tension across this boundary allowing more efficient diffusion. It is this fluid that may be brought up from the lungs to cause one to "foam at the mouth".
Mechanism of Breathing - Unlike the circulatory system that has an actual pump to move blood, the respiratory system lacks a pump to move air into and out of the lungs. Instead, the respiratory system relies on changes in air pressure to ventilate the lungs.
The lungs, resting in the thoracic cavity, are in a sealed chamber with only the tracheal tube leading to the outside. When size of the thoracic cavity is increased, its internal pressure decreases creating a situation in which the atmospheric air is at a higher pressure than the air in the lungs. Since the air will spontaneously move to areas of lower pressure, the external air will move into to the lungs to equalize the air pressure. We inhale. When the size of the thoracic cavity is decreased, its internal pressure becomes higher than the air surrounding the body. Air moves out of the lungs and we exhale.
The primary muscles that are responsible for changing the size of the thoracic cavity are the diaphragm and the intercostal muscles. The diaphragm is a sheet of muscle that stretches across the bottom of the thoracic cavity. In its relaxed condition, the diaphragm is somewhat dome-shaped, curving up into the thoracic cavity. When the diaphragm contracts, the muscle flattens out, expanding the thoracic cavity. The intercostal muscles are located between the ribs. When these muscles contract, the ribs are pulled upward and out, pushing the sternum forward. As with contraction of the diaphragm, contraction of the intercostal muscles increases the size of the thoracic cavity.
Provided the chest cavity remains intact, changes in the internal pressure caused by the movement of the diaphragm and intercostal muscles will cause air to move into and out of the lungs through the trachea. If a wound penetrates the chest cavity, instead of moving through the longer trachea tube, air will take the shorter route through the wound to equalize the air pressure. Rather than air moving into the lungs, the air then moves into the spaces surrounding the lungs. So even though the individual continues to try to breath by expanding and contracting their chest cavity, gas exchange in the lungs does not occur and the individual is at risk of suffocating.
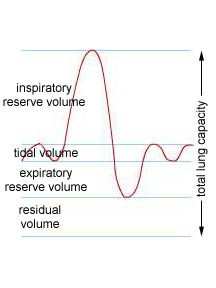
Gas Exchange in the Lungs - When we breathe we do not exchange all of the air within our lungs. In fact, during normal, resting breathing, we only exchange about 10% of air capacity of our lungs. This volume of air during normal, quiet breathing is called the tidal volume. On average, the tidal volume of an adult is about 500 ml.
|
If you breathe in as much air as you can, that volume above the amount you draw in during a normal breath is called the inspiratory reserve volume. On average this is about 3100 ml of air. If you exhale as much air as you can, the exhaled air in excess of what you normally exhale is called the expiratory reserve volume. This averages about 1200 ml. The maximum volume of air you can exchange by taking in as much air as possible and then exhaling as much air as possible is called the vital capacity. This is the sum of the tidal volume and two reserve volumes or about 4800 ml. Even when you exhale as much air as possible from your lungs, air remains in the lungs and passageways. This quantity of air is called the residual volume. It averages about 1200 ml giving the average total lung capacity to be about 6000 ml of air. |
 |
The air entering and leaving the lungs is a mixture of various gases. A convenient way to measure and express the quantity of any one particular gas within this mixture is to determine the partial pressure of the gas. One way you can think of partial pressure is as the pressure contribution by one gas to the total pressure of the mixture. Another way to think of it is as a measure of the concentration of a gas within a mixture. Partial pressure is usually expressed in millimeters of mercury (mm Hg). For example, the atmospheric pressure of the air around us is about 760 mm Hg. There is about 21% oxygen and about 0.04% carbon dioxide in the air (the rest, 79%, is mostly nitrogen). For these two gases, this corresponds to partial pressures of 0.21 x 760 = 160 mm Hg for oxygen and 0.0004 x 760 = 0.3 mm Hg for carbon dioxide. This is the composition of the air entering our respiratory system. The following table shows the average partial pressures of oxygen and carbon dioxide at various points in to body. The arrows show the net direction of gas movement. Note that for both oxygen and carbon dioxide, the net movement of these gasses is down a concentration gradient. The body must maintain this gradient if it is to maintain gas exchange.

Oxygen Transport - Despite the diffusion gradient, diffusion would not be an adequate mechanism to deliver and remove the respiratory gases from the cells throughout your body. One of the functions of the circulatory system is to speed the transport of these gases.
One of the problems with transporting oxygen in the blood is that oxygen does not dissolve well in water (recall that the blood's plasma is mostly water). In fact, only a little more than 1% of the oxygen that is carried by your blood is as a dissolved gas. To transport an adequate supply of oxygen, your circulatory system utilizes molecules called respiratory pigments. In mammals this molecule is hemoglobin.
Hemoglobin is a molecule consisting of four subunits. Each subunit contains an iron atom within what is called a heme group. Each heme group is able to bind to one molecule of oxygen allowing each molecule of hemoglobin to carry up to four oxygen molecules. Hemoglobin molecules are located within the red blood cells of the blood. Its been estimated that each red blood cell contains some 265 million molecules of hemoglobin.
|
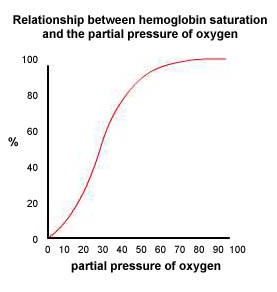
Hemoglobin is something like an oxygen magnet and, like a magnet, the oxygen will be attracted to and attached to the hemoglobin depending on what other attracting forces there are in the immediate environment. One of these other attracting forces in the environment is the partial pressure of oxygen surrounding the hemoglobin molecule. When the surrounding partial pressure of oxygen is relatively high, above 100 mm Hg for example, the hemoglobin molecules are nearly saturated with all the oxygen they can carry. As the partial pressure of oxygen decreases, oxygen is pulled away from the hemoglobin, first weakly but then much more strongly as the partial pressure drops below 40 mm Hg. Note that in our bodies, as the blood leaves the lungs where the partial pressure of oxygen is about 105 mm Hg, the hemoglobin will be nearly 100% saturated with oxygen. It can carry no more. When the blood is in the body tissues where the partial pressure is about 40 mm Hg, the hemoglobin will give up about 30% of its oxygen, hanging on to the other 70%. This oxygen still attached to the hemoglobin acts as a reserve. Should you begin some physical exertion, more of this oxygen attached to the hemoglobin will be given up as the partial pressure of oxygen in the tissues decreases. |
 |
If the partial pressure of oxygen drops below 20 mm Hg, a second reserve of oxygen begins to be released. This oxygen comes from another respiratory pigment located in muscle called myoglobin. If the oxygen reserves are consumed, the tissues can temporarily switch to anaerobic cellular respiration, producing lactic acid instead of carbon dioxide as a waste product.
Other environmental factors that can influence how much oxygen hemoglobin can carry include the acidity of the environment (hemoglobin will hold less oxygen at lower pHs, this is called the Bohr effect), the partial pressure of carbon dioxide (low concentrations of carbon dioxide lead to less oxygen attached to hemoglobin) and temperature (less oxygen is attached to hemoglobin at higher temperatures).
There is also a difference between the hemoglobin found in a fetus from that found in an adult. Fetal hemoglobin has a higher affinity for oxygen than does adult hemoglobin. This allows the fetal hemoglobin to take oxygen from the mother's blood, providing the developing fetus with needed oxygen.
Carbon Dioxide Transport - Unlike oxygen, carbon dioxide dissolves somewhat more easily in the plasma and so one finds a greater percentage as a dissolved gas (about 7% of the carbon dioxide transported by the blood is as a dissolved gas). In addition, some carbon dioxide can complex with hemoglobin and is transported as a hemoglobin/carbon dioxide complex (carbaminohemoglobin). About 23% of the carbon dioxide transport occurs in this fashion.
The majority of carbon dioxide transport occurs as ions dissolved in the red blood cells and the blood's plasma. When carbon dioxide dissolves in water, it will spontaneously react with the water molecules in a reversible reaction to form carbonic acid. The carbonic acid, in turn, will spontaneously break down in a reversible reaction to produce bicarbonate ions and hydrogen ions. These hydrogen ions will make the solution more acidic.
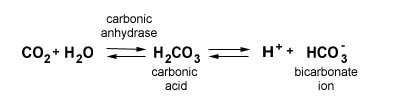
While the reaction to form carbonic acid from water and carbon dioxide molecules will occur spontaneously (when exposed to the air, pure distilled water will become slightly acidic due to these reactions) your body speeds this process with the enzyme carbonic anhydrase located within the red blood cells.
Whether this reaction proceeds to the right to produce more bicarbonate ions are proceeds to the left to produce carbon dioxide depends on the partial pressure of carbon dioxide. When the surrounding partial pressure of carbon dioxide is high, the net result of these reactions is covert carbon dioxide into bicarbonate ions. When the surrounding partial pressure of carbon dioxide is low, the net result is to produce carbon dioxide from bicarbonate ions and hydrogen ions. In the capillaries in your body tissues, the reaction will proceed to the right. In your lungs, the net results of the reaction will proceed to the left.
Regulation of Respiration - The objective of the respiratory system is to maintain the proper levels of oxygen and carbon dioxide in the body's tissues and circulatory system. To do this, the body monitors the levels of these gases in the circulatory system and adjusts the respiratory system to try to maintain the homeostatic levels.
The principle mechanism you body uses to regulate the respiratory system is to monitory the blood's pH. Since much of the carbon dioxide in your blood is transported as bicarbonate and hydrogen ions, carbon dioxide in the blood will alter the blood's pH (pH is a measure of the hydrogen ion concentration in a fluid). As more carbon dioxide enters the blood, more bicarbonate and hydrogen ions are formed, lowering the blood's pH, making it more acidic. When carbon dioxide leaves the blood, the pH increases and the blood becomes less acidic.
Chemoreceptors located within the brain and aorta monitor the blood's pH. The normal homeostatic pH level of the blood is about 7.4. If more carbon dioxide enters the blood, these pH values begin to fall. These chemoreceptors detect this change and trigger the inspiratory center in the medulla oblongata to signal the diaphragm and intercostal muscles to contract more forcefully and more frequently. This results in your body exhaling more carbon dioxide that ultimately results in the blood's pH returning to normal. If the blood's pH becomes too high, just the opposite occurs. The inspiratory center slows the rate of breathing, more carbon dioxide is retained within the body and the pH return to homeostatic conditions.
One can consciously override this mechanism of control. If one hyperventilates, they end up expelling too much carbon dioxide from their body and the blood's pH increases. If this continues it will lead to nervousness, muscle spasms, convulsions and ultimately death. A simple measure to treat this problem is to have the person breath into a paper bag. The carbon dioxide content in the bag increases as they rebreathe the air helping the system to lower the blood's pH.
If one holds one's breath or is prevented from breathing, carbon dioxide will accumulate in the blood, lowering the blood's pH. This leads to respiratory acidosis. An individual with respiratory acidosis becomes disoriented and may pass out. Prolonged acidosis leads to coma and death.
Your body also contains chemoreceptors that directly measure the levels of oxygen and carbon dioxide in you blood. However these are of secondary importance in regulating your day to day breathing under normal conditions.